New Era Partners has performed many Supply Chain X-Rays and Workshops with our food distribution clients. We typically kick off our client engagements by auditing their current software systems and processes as part of an internal gap assessment. This helps us determine if the processes our clients currently have in place meet expectations required by the FDA Food Traceability Rule (FSMA 204), such as:
- The ability to receive and ingest suppliers’ traceability records, or Key Data Elements (KDEs).
- The ability to store KDEs for 2+ years, and to share those KDEs with customers using methods that meet customer requirements.
- The ability to generate an Electronic Sortable Spreadsheet upon FDA’s request.
We start by asking…
What System(s) Does Your Company Use to Create, Capture, Store, and Share Traceability Records (KDEs)?
One of the most eye-opening discoveries the New Era Partners consulting team has experienced while working with clients is the number of disparate systems used to capture, store and share the myriad of traceability data that enterprise organizations are required to track. Just about every large distribution center has some combination of the following systems:
- MDM – Master Data Management
- ERP – Enterprise Resource Planning
- WMS – Warehouse Management System
- Payment Processing
- Voice Picking
While these systems are great at managing the data and processes they are each designed to manage, they have yet to prove to be effective at providing a full FSMA 204 compliance solution on their own. For example, none of these can capture and store the Traceability Lot Code (TLC) and the Traceability Lot Code Source from every pallet arriving from every supplier. And none of these systems seamlessly talk to each other so that data can easily be shared between them. While integrations can be set up or manual processes put into place, it is often more cost-effective and efficient to invest in software that is designed to meet FSMA 204 compliance requirements.
Continue reading to better understand the rule requirements and how your current systems and processes may or may not help your organization meet those requirements. If you haven’t yet familiarized yourself with the rule, take a minute to read our helpful guide on “How to Read the FDA FSMA 204(d) Final Food Traceability Rule”.
Key Questions to Ask Regarding Systems and Processes Currently Used in Your Distribution Center
1. Are your suppliers already sharing KDEs with you, and if so, is the data in a format your systems can ingest?
When auditing internal systems and processes, ask if your suppliers are already sharing their KDEs with you in a format your systems can ingest. We’ve found that while many of our distributor clients are receiving traceability data from their suppliers, the current systems they use to receive shipments cannot easily ingest the data. Even if 100% of your suppliers share their shipping KDEs with you, your organization would be disrupting your supply chain’s ability to comply with FSMA 204 if your internal systems can’t ingest the data.
We recommend a FSMA 204 compliance solution, like iFoodDS Trace Exchange™ with IBM Food Trust™, that can ingest data in a variety of methods, such as API, ASN/EDI, flat file (csv), third-party application connections, or manual entry from documents such as Bill of Ladings.
Ingesting Data Using Existing Processes
You may already have processes in place that enable easy data capture. For example, suppose your suppliers use PTI-compliant case labels with GS1-128 barcodes like the one below; you can capture just about all the traceability data you need to meet both shipping and receiving KDE requirements. The rest of the data can be found on your Bill of Lading.
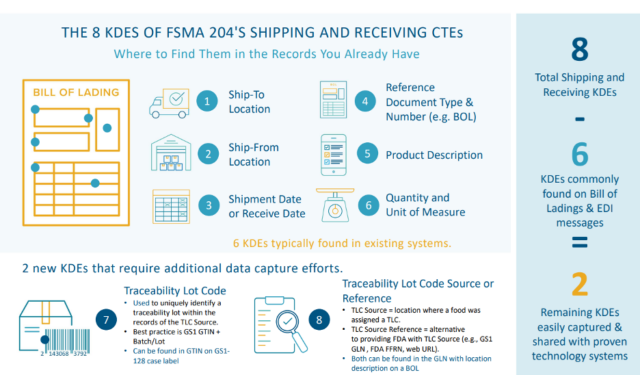

2. Are You Capturing the Correct FSMA 204 Supplier Data at Picking for Mixed Pallets
Suppose your systems can ingest the required shipping KDEs from your suppliers. Next, you’ll want to analyze your picking process to ensure you can accurately track the TLCs from the mixed pallets you receive, and the products picked to make up your mixed pallets that will ship to your customers.
Below is a list of questions we ask our clients to understand their picking process better and identify where in that process you can identify and record your Traceability Lot Code and Traceability Lot Code source. Think about how your own organization would respond.
- Pallets may have more than one supplier lot code. What process do you use in your DC to separate lot codes? If you aren’t separating them now, you will need to do so to comply with FSMA 204.
- Do your pickers use Voice Pick technology, like Vocollect, that tells them what to pick and where?
- When picking, do you pick by sku, by lot code, or some other unique way?
- Do you pick a pallet to zero, or do you pick to the bottom row, pull another pallet from storage, then put the product from the previous pallet on top of the new pallet? We’ve found that picking to the bottom row is most common among our clients.
- Do you use the voice pick numbers on the PTI-compliant labels to choose what items to pick and add to the mixed pallet for your customer? See example.

Understanding the picking processes used in your DC will help you know how you can identify the Traceability Lot Code (TLC) and the Traceability Lot Code (TLC) source. You will need this information as part of the shipping Critical Tracking Event (CTE) recordkeeping requirements. This information will also need to be shared with your customers.
3. Are You Able to Send FSMA 204 Compliant Shipping Records?
Let’s assume you capture all required data from 100% of your supplier network. Your picking systems and processes can capture the necessary data on the mixed pallets you’ve built to send to your customers – can you share that data with your customers in a format that their systems can ingest? Do you need to send shipping records to upstream suppliers? Have your customers and upstream suppliers communicated their specific requirements to you, and can you meet those requirements?
Traceability records can be shared in a variety of methods, such as:
- Flat files (csv) sent via a secure, electronic method.
- API
- ASN
- Other 3rd party connection
- Manual data entry from existing forms, such as Bill of Ladings
Your customer may already request that you send your records using a specific method, or they may be planning to implement a network traceability system so they can easily and securely ingest and store data from all their suppliers consistently. It’s helpful to understand your customers’ requirements early on to understand the bigger picture and how each customer in your network may be forming their own FSMA 204 compliance plan. Understanding their plan can save you from making a decision that will cost you more later.
4. Are There Other Scenarios to Consider That May Apply to Your Distribution Center?
Through our consulting experience, we have found that many distributors will provide other services beyond just receiving whole pallets of products, breaking down those pallets, and sending out new, mixed pallets. Depending on customer needs, supplier issues, and opportunities in the local market, distributors may provide other services that could complicate your supply chain’s ability to comply with FSMA 204. For example:
Local Grower Programs:
Many DCs will add a seasonal local grower program where they act like the “Initial Packer” for many local growers. If you perform this service, you are also responsible for Initial Packing KDEs, including creating a Traceability Lot Code for that product. Can your internal systems and processes accommodate this requirement?
Repacking Transformation:
Your DC may repack a product for a private label deal or a product that requires culling. If you are sorting and packing a product from one case to another, this is considered Transformation, requiring another set of KDEs to be recorded. Can your internal systems and processes accommodate this requirement?
Processing:
Many DCs have added a processing line to create value-added products like fruit cups, shucked corn, trimmed produce, or cold-pressed juice. If the processing does not include a final kill step, then this is considered Transformation, which will require another set of KDEs to be recorded. Can your internal systems and processes accommodate this requirement?
5. What Traceability Records Need to be Included in an Electronic Sortable Spreadsheet within 24 hours of FDA’s Request?
One of the most critical requirements of the FDA’s Food Traceability Rule, FSMA 204, is the expectation that your organization will generate and provide the FDA with an electronic sortable spreadsheet within 24 hours of their request.
We recently posted an article that dives deeper into this topic: “FDA Has Just Asked for an Electronic Sortable Spreadsheet – Now What?” One of the questions we are asked most often is how to automate this process so that you aren’t scrambling to pull massive amounts of data from disparate systems when that request from the FDA comes in just before the weekend.
The example below shows where you might have to pull records from to create your electronic sortable spreadsheet if you still use paper and other disparate systems to record and store your traceability records.
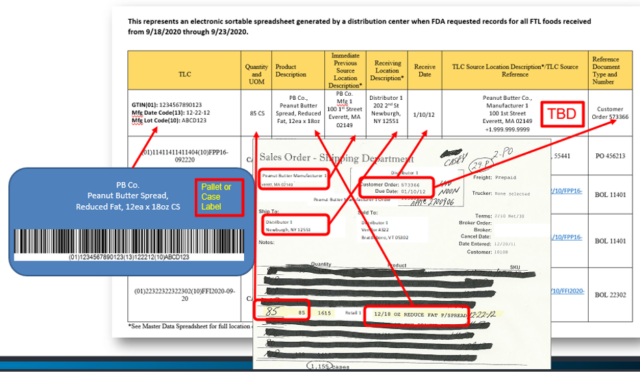
The Produce Traceability Initiative (PTI) has provided some great examples of Electronic Sortable Spreadsheets you may want to reference.
Conclusion
It should become clear at this point how critical it is to have systems in place that can not only securely capture, store, and share required Key Data Elements with your customers and other trading partners but can also generate an electronic sortable spreadsheet quickly and accurately. Ensure that if you are pulling from multiple systems, you know what data is stored where and who has access to what. It is best practice to eliminate as much manual work as possible to avoid accuracy issues and delays.
Once you’ve completed your systems and process audit and addressed all of the topics above, determine your confidence level in your organization’s ability to be fully FSMA 204 compliant. Does it make sense to stick with your existing processes or to consider a fully compliant solution that enables you to:
- Securely capture, and store Key Data Elements (KDEs) essential for FSMA 204 compliance.
- Effortlessly share KDEs with your customers, enhancing transparency and trust in your food supply chain.
- Choose the data sharing method that works best for your organization, including API, ASN, flat file or other third-party application connections.
- Easily connect with your suppliers when necessary to receive the Key Data Elements (KDEs) required for FSMA 204 compliance.
- Be confident in the accuracy of your data and your readiness to meet regulatory requirements.
- Store and manage your organization’s master data and documents needed for compliance.
- Quickly generate an electronic sortable spreadsheet with exact data needed by FDA for FSMA 204 compliance.
- Create and access reports and dashboards for full traceback and traceforward visibility.
- Receive technical support and online training when you need it.
If all this still feels overwhelming, our FSMA 204 experts at New Era Partners are here to help. Our various services are aimed at identifying where you’re at in your FSMA 204 compliance journey and getting you on the right path.


