Look ahead beyond January 20, 2026: The compliance deadline for FDA’s Food Traceability Rule (FSMA 204) has passed, and your organization is now expected to be compliant with the rule. One of the foods that you manufacture, process, pack, or hold is on the Food Traceability List (FTL) and is suspected to be affiliated with a current outbreak. FDA just asked for an Electronic Sortable Spreadsheet. What, exactly, are they looking for?
A Key Deliverable of FSMA 204
One of the key deliverables is that the Food and Drug Administration (FDA) can request a list of your suppliers who supply products on the FTL that you have packed, transformed, shipped, and/or received over a specified period of time, in addition to applicable Key Data Elements (KDEs) for any of those events. When public health is threatened, FDA may request that information be provided in the form of an Electronic Sortable Spreadsheet within 24 hours of the request.
You must be able to provide that information no matter what day or time the request is made, and chances are probably good that it will come in at 5:00 pm on a Friday! So, you get that call from the FDA asking for outbreak traceability records in an Electronic Sortable Spreadsheet within 24 hours, which happens to be Saturday at 5:00 pm. Now what?
Why is the FDA requesting an Electronic Sortable Spreadsheet within 24 hours?
Before we dive into exactly what data or records are needed in the Electronic Sortable Spreadsheet to comply with FSMA 204 requirements, it’s important to understand the “WHY.”
The answer can be found in the rule: To help FDA prevent or mitigate a foodborne illness outbreak, or to assist in the implementation of a recall, or to otherwise address a threat to public health, including situations where FDA has a reasonable belief that an article of food presents a threat of serious adverse health consequences or death to humans or animals.
FDA is developing a new Product Tracing System (PTS) to receive and analyze the industry’s traceability data and more effectively and rapidly trace food within the U.S. Organizations across the supply chain will be able to upload their Electronic Sortable Spreadsheets or other traceability records into this new system (or have FDA do it for them) and FDA will have the traceability data they need at their fingertips.
This new PTS will:
- Improve traceback and trace forward analysis
- Encourage government and industry collaboration
- Assist with root cause analysis
- Contribute to other illness prevention efforts
Watch this 8-minute video to learn more about how FDA will use the PTS to more quickly ingest and analyze your traceability records and conduct more efficient, expedient outbreak investigations.
What data is the FDA is requiring in the Electronic Sortable Spreadsheet as part of FSMA 204 compliance?
The FDA reviewed and commented on thousands of pages of questions and concerns from the industry about what is expected of organizations across the fresh food supply chain for compliance with FSMA 204. One of the biggest questions was what data the FDA will expect retailers and restaurants to provide via an Electronic Sortable Spreadsheet within 24 hours of the request.
In September 2022, the FDA posted a white paper called “Retail food establishments (RFEs) and restaurants: What records do I need to keep for the Food Traceability Rule?” In the document the following was outlined:
Recordkeeping requirements under the rule depend on the activity (Critical Tracking Event or CTE) being conducted by the regulated entity. Generally, RFEs and restaurants must maintain receiving records as described in 21 CFR § 1.1345. The following key data elements (KDEs) must be maintained and linked to the traceability lot for the food:
- Traceability lot code for the food
- Quantity and unit of measure of the food
- Product description for the food
- Location description for the immediate previous source of the food
- Location description for where the food was received
- Date you received the food
- Location description for the traceability lot code source, or the traceability lot code source reference
- Reference document type and reference document number
Of course, it’s not just grocery retailers and foodservice operators who are responsible for providing their traceability records in an Electronic Sortable Spreadsheet upon request. All organizations across the supply chain who manufacture, process, pack or hold foods on the FTL will be responsible for providing KDEs for each CTE they are responsible for.
And all those KDEs for each CTE must be included on that spreadsheet for each FTL food you produce (or as requested by FDA), along with any other information needed to understand the information in the spreadsheet.
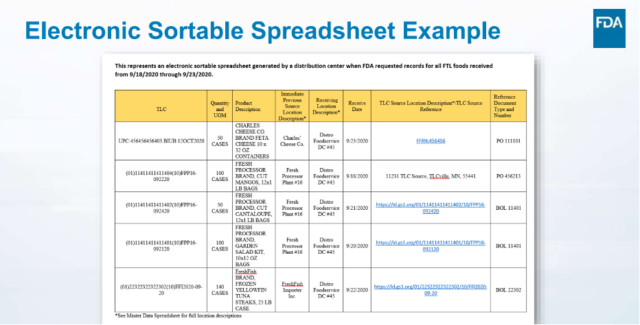
Example of a FSMA 204 Electronic Sortable Spreadsheet:
When the New Era Partners Consulting Team works with our clients to assist them through Supply Chain X-Rays and FSMA 204 Team Workshops, we always leave behind examples of how they can create their own working FSMA 204 Compliance Electronic Sortable Spreadsheet, like this example from a 2023 FDA presentation on FSMA 204:
In another example we can take data received from case labels and sales orders and see what data would go where in the Electronic Sortable Spreadsheet:
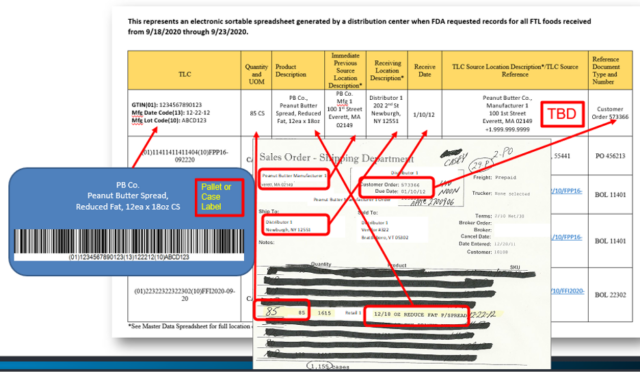
Nearly all of the data you need to provide can be found on your case or pallet labels (if following Produce Traceability Initiative (PTI) and GS1 Serial Shipping Container Code (SSCC) labeling standards) and your sales order, or bill of lading. The Traceability Lot Code “Traceability Lot Source” location description/TLC source reference are the only data points not found on case or pallet labels, or sales orders. Your organization may need to update your processes to include the TLC location description or source reference in your data records, such as on your Bill of Ladings.
How can you generate the Electronic Sortable Spreadsheet using internal data systems?
Much of the data needed to populate the electronic sortable spreadsheet can be found within your organization’s existing internal data management systems, such as:
- MDM – Master Data Management
- ERP – Enterprise Resource Planning
- WMS – Warehouse Management System
- ASN – Advanced Shipping Notice
- EDI – Electronic Data Interchange
If you have solid traceability plans and recordkeeping processes in place, having accurate data shouldn’t be an issue. Knowing where that data is stored, which may be in disparate systems and managed by different users or departments, can be the real challenge. This can make compiling all that data into one spreadsheet an onerous and time-consuming endeavor.
We recommend auditing your systems to document where data is stored, and to identify gaps in data storage. If your current systems don’t enable you to capture, store, and share all required KDEs, you may need to look into replacing existing systems with one(s) that will do this for you. If you do find yourself needing to go down that path, check out this helpful checklist for finding the right solution partner, or contact New Era Partners for guidance.
Investigations Can Take Months
To place what you’ve just learned about electronic spreadsheets in context, let’s remind ourselves of how outbreak investigations work now, and why getting those KDEs to FDA quickly is so critical.
FDA’s Traceback of Foodborne Illness Outbreaks
- Conduct Patient Interviews: The Centers for Disease Control and Prevention (CDC), and state and local health departments, work with patients to link illnesses to a specific food. If FDA-regulated products are suspected, the FDA will initiate a traceback investigation.
- Follow Up at Points of Exposure: FDA, CDC, and state and local partners visit locations where ill people report eating or purchasing food and look at their menus, ingredients, and supply chain records.
- Mapping the Food’s Transportation and Distribution Paths: FDA then works backward to identify where and how the food made its way to the consumer.
- Inspecting Facilities Where Food Processing Took Place: Any ingredient, or the food processing itself, could be the source or cause of the contamination or illness.
- Going Back to the Source: Depending on what the traceback information reveals, FDA may go back to the source of the ingredients to conduct an inspection.
- Determining a Relationship Between Environmental and Product Samples and the Outbreak Pathogen: FDA may collect environmental and product samples at any stage in the process. If the sample tests positive for a pathogen, FDA will determine the relationship of these samples to the outbreak pathogen.
- Issuing a Public Advisory: To protect public health, the FDA will issue a public health advisory for investigations that have resulted in specific actionable steps for consumers to take to protect themselves.
- Preventing Future Outbreaks: Companies may need to identify and implement corrective actions.
CDC’s 7-Step Process for Investigating Multistate Foodborne Outbreaks
- STEP 1/Detect: Detect a possible outbreak by monitoring for reported illnesses nationwide.
- STEP 2/Find: Define who will be included in the outbreak and look for additional sick people.
- STEP 3/Generate: Generate hypotheses (potential explanations) by interviewing people about what they ate before getting sick.
- STEP 4/Test: Test hypotheses by comparing what sick people ate to what people who are not part of the outbreak ate.
- STEP 5/Solve: Confirm the contaminated food using epidemiologic, laboratory, and traceback information and identify the point of contamination.
- STEP 6/Control: Stop the outbreak by recalling contaminated food, cleaning or closing food facilities, and providing advice to people and businesses.
- STEP 7/Decide: Decide the outbreak is over when illnesses stop and the contaminated food is no longer available.
The FDA and CDC outbreak investigation process can take weeks, months, or well over a year in some cases. The goal of gathering traceability data in an Electronic Sortable Spreadsheet is to enhance the speed and accuracy of these investigations. Every day that the source of the outbreak is not identified, contaminated products remain on retail shelves, on restaurant menus, and in consumers’ homes, leading to the consumption of more contaminated products and consumer health risks.
Conclusion:
We’ve consulted with many major grocery retail and foodservice companies and have found during our x-rays and workshops that there is a need for one source of truth for traceability data. With one source of truth, companies won’t be scrambling to pull data from disparate systems, trying to remember where each piece of data is located. An Electronic Sortable Spreadsheet will be that one source of truth, giving you confidence that the data that may be requested by the FDA is current, accurate and all in one place.
Impartial, expert support is available from consultants like New Era Partners. When the FDA knocks on your door at 5:00 pm on a Friday afternoon, you and your traceability team want to feel confident that you have all the required data at your fingertips.
We can help you in your journey to get there.