Your company already has a traceability or recall plan as part of your Good Manufacturing Practices (GMPs), but you are now covered by the new FDA Food Traceability Rule, FSMA 204. This rule requires that companies manufacturing, processing, packing, or holding foods on the Food Traceability List (FTL) have a plan that outlines how they will meet additional recordkeeping requirements.
So, what exactly do you need to add to create a traceability plan that meets the requirements of this rule by the January 2026 compliance deadline? The good news is that you’re not starting from scratch but building on what you already have. Here is a step-by-step look at what you’ll need to add to your plan.
Traceability Plan Requirements § 1.1315
In order to understand what we must add to our traceability plan to be compliant, we need to first understand what the rule is requiring; below is the actual language in the rule:
1.1315 What traceability plan must I have for foods on the Food Traceability List that I manufacture, process, pack, or hold?
(a) If you are subject to the requirements in this subpart, you must establish and maintain a traceability plan containing the following information
(1) A description of the procedures you use to maintain the records you are required to keep under this subpart, including the format and location of these records.
(2) A description of the procedures you use to identify foods on the Food Traceability List that you manufacture, process, pack, or hold;
(3) A description of how you assign traceability lot codes to foods on the Food Traceability List in accordance with § 1.1320, if applicable;
(4) A statement identifying a point of contact for questions regarding your traceability plan and records; and
(5) If you grow or raise a food on the Food Traceability List (other than eggs), a farm map showing the areas in which you grow or raise such foods.
(i) Except as specified in paragraph (a)(5)(ii) of this section, the farm map must show the location and name of each field (or other growing area) in which you grow a food on the Food Traceability List, including geographic coordinates and any other information needed to identify the location of each field or growing area.
(ii) For aquaculture farms, the farm map must show the location and name of each container (e.g., pond, pool, tank, cage) in which you raise seafood on the Food Traceability List, including geographic coordinates and any other information needed to identify the location of each container.
(b) You must update your traceability plan as needed to ensure that the information provided reflects your current practices and to ensure that you are in compliance with the requirements of this subpart. You must retain your previous traceability plan for 2 years after you update the plan.
Let’s break down each requirement of the Traceability Plan:
1.1315(a)(1) A description of the procedures you use to maintain the records you are required to keep under this subpart, including the format and location of these records.
The list of all the required records for Initial Packing are listed in 21 CFR 1.1330. The overall expectation from the new FDA Traceability Rule is that all traceability data the FDA requests must be provided in a sortable spreadsheet within 24 hours.
Suppliers of food on the FTL must document and maintain procedures and records of the following:
- How all the records are created:
- Who, or what position(s), is responsible for creating each record?
- Where is each record created?
- Handwritten in the field or on the facility floor?
- Placed on boxes or bins prior to or after harvest?
- ERP System, WMS, or some other system(s)?
- When is each record created?
- How all the records are managed:
- What systems are used to manage the records?
- Who, or what position(s), is responsible for managing each record?
- Where all the records are stored:
- What systems are used to store the records?
- Who has access to this storage area and/or systems?
- Knowing you have 24 hours to provide the FDA a sortable spreadsheet with all the traceability data, who would you need to call at 5 p.m. on a Friday to get access to everything?
1.1315(a)(2) A description of the procedures you use to identify foods on the Food Traceability List that you manufacture, process, pack, or hold;
Many companies in the supply chain can easily look at the FTL and instantly know they have an FTL product, like leafy greens, sprouts, seafood, etc. Many other companies, such as distributors, may need to rely on their suppliers to let them know. All companies in the food supply chain have to create a process and procedures to identify whether they grow, pack, process, cool, ship and/or receive products on the FTL list.
Some key questions to ask yourself while creating the procedures are:
- Do you process a product, not on the FTL, that through transformation becomes an FTL product?
- Example: Carrots are not on the FTL, but fresh cut carrots are.
- What procedures do you use to make sure your suppliers notify you that they are sending you a product on the FTL?
- How do you notify your clients you are sending them a product on the FTL?
1.1315(a)(3) A description of how you assign traceability lot codes to foods on the Food Traceability List in accordance with § 1.1320, if applicable;
To comply with this subpoint we need to understand what is written in CFR § 1.1320:
1.1320 When must I assign traceability lot codes to foods on the Food Traceability List?
(a) You must assign a traceability lot code when you do any of the following: Initially pack a raw agricultural commodity other than a food obtained from a fishing vessel; perform the first land-based receiving of a food obtained from a fishing vessel; or transform a food.
(b) Except as otherwise specified in this subpart, you must not establish a new traceability lot code when you conduct other activities (e.g., shipping) for a food on the Food Traceability List.
Below are some questions to ask if you are creating Traceability Lot Codes (TLC):
- What is your corporate process for creating and assigning lot codes?
- Do you use PTI, GS1, or some other process for creating lot codes?
- How do you make sure the lot codes are assigned to the correct product?
- How do you make sure the label identifying the product and lot code does not end up on the wrong cases and/or pallets?
- Internal audits?
- Checking at shipping dock?
- How do you make sure the label identifying the product and lot code does not end up on the wrong cases and/or pallets?
- What is the process for assigning lot codes to products that are reworked?
1.1315(a)(4) A statement identifying a point of contact for questions regarding your traceability plan and records; and
When putting together the FSMA 204 compliance team, you will need members from different parts of your operation, such as Food Safety & Quality, IT, Operations, Supply Chain, Procurement, Legal & Regulatory. So, do you want one key person or position as the key contact and/or the team be the contact?
- The point of contact name and/or named position in the org chart.
- Name
- Position(s) – “Traceability Plan Team”
- Email: TraceabilityPlan@corp.com
1.1315(a)(5) If you grow or raise a food on the Food Traceability List (other than eggs), a farm map showing the areas in which you grow or raise such foods.
(i) Except as specified in paragraph (a)(5)(ii) of this section, the farm map must show the location and name of each field (or other growing area) in which you grow a food on the Food Traceability List, including geographic coordinates and any other information needed to identify the location of each field or growing area.
(ii) For aquaculture farms, the farm map must show the location and name of each container (e.g., pond, pool, tank, cage) in which you raise seafood on the Food Traceability List, including geographic coordinates and any other information needed to identify the location of each container.
In creating the required farm map with its field names and geographic coordinates, there are some factors to keep in mind:
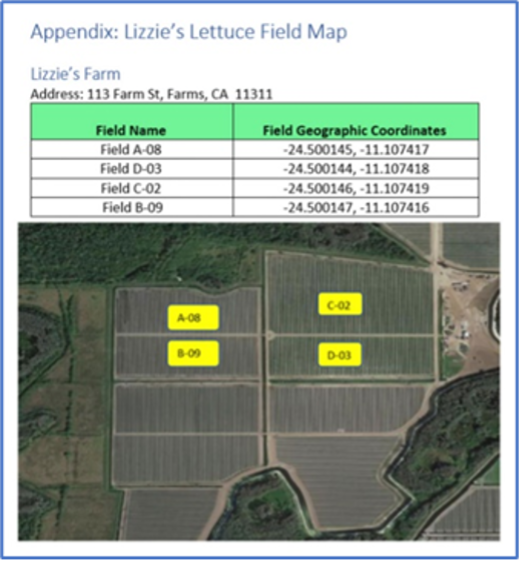
- The farm and field names are Key Data Elements (KDE) used in Harvest Critical Tracking Events (CTEs) and Initial Pack CTEs. They must match in the CTEs to enable FDA to trace back from the Initial Pack records to the farm and field.
- The farm address must be the physical address of the farm.
- Farm Name
- Address Line 1
- Address Line 2
- City
- State (Region)
- Zip (Postal Code)
- Country
- Telephone
- GLN {optional}
- Field/Lot Name {could be GLN extension}
- Field/Lot Geographic Coordinates
- If your farm map is documented as part of other food safety plan requirements your organization has in place, you may note where those farm map records can be found in lieu of including a copy as part of your Traceability Plan.
1.1315(b) You must update your traceability plan as needed to ensure that the information provided reflects your current practices and to ensure that you are in compliance with the requirements of this subpart. You must retain your previous traceability plan for 2 years after you update the plan.
To ensure that your plan is current, consider this:
- How often will the FSMA 204 Traceability team review your traceability plan?.
- This could be an annual review, like reviewing your food safety procedures. Also, anytime a process is updated throughout the year the traceability plan will need to be updated as well.
- Who can make changes to the plan, who reviews and signs off on the changes?
- How do you version control all changes to the plan?
- If you have a version control process list, where are you keeping that process?
- How do you verify and validate that the plan truly reflects what is happening in the field and/or the floor?
- Are you performing internal inspections of the processes?
- How do you manage customer complaints of data not matching products sent?
- What is the corrective action process when issues arise?
- Who is in charge of managing the corrective actions?
- How do you make sure all the records are NOT deleted prior to the mandated 2 years?
- Who manages the systems, and do they know the protocols for deleting records?
- What happens if records are deleted prior to 2 years?
- What are corrective Actions?
Building your traceability plan is a great way to evaluate your current traceability processes and identify gaps in your path to compliance. If you need guidance in putting together your traceability process and building out your traceability plan, New Era Partners is here to help. We offer a variety of consulting services to fit the needs of any organization in the fresh food supply chain.


